Background:
Chemotherapy in combination with radiotherapy (RT) is the standard for early-stage (ES) Hodgkin lymphoma (cHL). Despite high cure rates (12-year overall survival (OS) >93%), there is room to improve short and long-term outcomes for children and adults with ES cHL. Incorporation of immunotherapy (IO) into front line treatment of ES cHL is an opportunity to both improve progression-free survival (PFS) and maintain OS, while minimizing long-term morbidity and mortality by reducing exposure to RT and high-dose chemotherapy.
Study Design and Methods:
HL leaders of the NCTN groups, expert HL researchers and physicians, and representatives from the Cancer Therapy Evaluation Program (CTEP) formed a working group in April 2020 with the goals of harmonizing treatment approaches for ES cHL across pediatric and adult patients and reaching consensus regarding the optimal study design for incorporating IO therapy into frontline treatment. Study champions identified from each of the North American cooperative groups [Children's Oncology Group (COG), SWOG, ECOG-ACRIN, Alliance, NRG] and experts in imaging, radiation oncology, lymphoma biology and patient-reported outcomes (PROs) were included. The resulting COG-led clinical trial, AHOD2131, represents the largest ES cHL trial in the history of North American cooperative groups and the first to enroll patients across the age continuum.
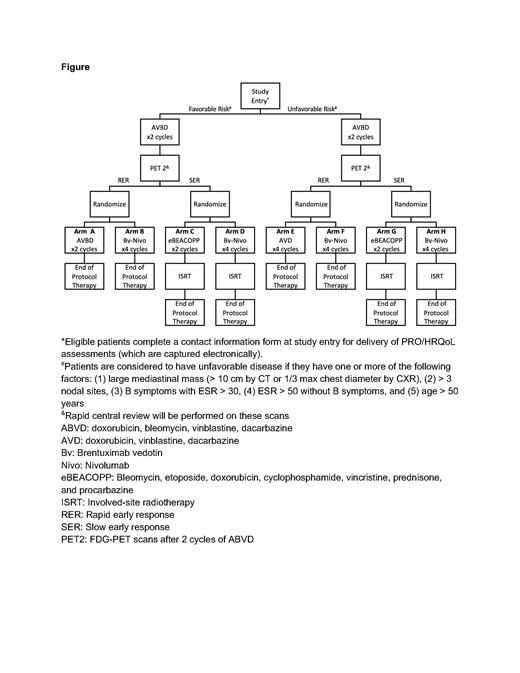
AHOD2131 (NCT05675410; Figure) is a randomized, phase 3 trial enrolling patients ages 5 to 60 years with newly diagnosed, previously untreated stage I and II cHL and will investigate the addition of the CD30-antibody drug conjugate brentuximab vedotin (Bv) with PD-1 blockade (nivolumab) compared to standard chemotherapy +/- RT. Since the April 2023 activation, 97 North American sites have activated the trial, and 21 participants have enrolled. Target enrollment is 1875 patients over 5 years of accrual, for an estimated 1782 eligible and evaluable patients.
The primary objective is to compare the 3-year PFS of patients with ES cHL treated through a response-adapted, superiority design with either standard therapy or with an IO approach (BV + nivolumab). All patients will be stratified as favorable or unfavorable based on disease risk features at enrollment. Based on initial response assessment by PET/CT (via central review) after 2 cycles of ABVD, patients were classified to PET2 positive (SER, defined as 5 Point Score 4 or 5, ~15% of patients) and PET negative (RER). Those who are PET positive will receive involved site RT (ISRT). The SER and RER patients will be randomized to standard chemotherapy vs. IO therapy respectively.
In addition to the primary outcome of PFS, there are 11 secondary and 10 exploratory aims. The secondary aims focus on OS, PFS of subgroups, and event-free survival at 12 years, physician-reported treatment-related adverse event (AE) rates, patient-reported AE using pediatric and adult versions of Patient-Reported Outcomes version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE), and the capture of representation by self-report and the use of electronic PRO data collections.
The exploratory aims include the assessment of imaging central review as compared to local institutional review and the use of convolutional neural networks through artificial-intelligence machine learning. The study will examine the effect of therapy on acute and long-term adverse effects including health-related quality of life (HRQoL) and OS for up to 12 years. The study will compare patient self-report and provider AE reports and evaluate the associations of self-reported race/ethnicity and social determinants of health (SDOH)and post-progression/post-relapse OS.
AHOD2131 strengthens the effort between the North American cooperative groups to conduct collaborative clinical trials and aims to harmonize an improved standard of care for ES cHL across the age continuum.
Disclosures
Keller:Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific advisory council. Hoppe:Merck SAC for AHOD1822/KN667 low risk: Consultancy. Roth:Roche: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Consultancy, Membership on an entity's Board of Directors or advisory committees. Parsons:Seagen: Consultancy. LaCasce:Research to Practice: Consultancy; Seagen, Kite Pharma: Membership on an entity's Board of Directors or advisory committees. Grover:Sangamo: Current holder of stock options in a privately-held company; Novartis: Honoraria; Tessa Therapeutics: Research Funding; Caribou Biosciences: Honoraria; Seagen: Honoraria; Genentech: Honoraria; Kite: Honoraria; ADC Therapeutics: Consultancy, Honoraria; Seattle Genetics: Consultancy. Allen:Kyowa Kirin: Consultancy; Daiichi Sankyo: Consultancy; Seattle Genetics: Consultancy; Secura Bio: Consultancy. Evens:ORIEN, Leukemia & Lymphoma Society.: Other: grant/research support, Research Funding; Novartis, AbbVie, Pharmacyclics, Seattle Genetics, Hutchmed, Incyte, Daiichi Sankyo, Epizyme; Curio, Cota, Patient Power, Curio Science, OncLive, Research to Practice: Consultancy. Schoder:Aileron Therapeutics: Honoraria. Castellino:Bristol Meyers Squibb: Honoraria, Other: Scientific Advisory Committee; SeaGen Inc.: Other: Scientific Advisory Committee - No honoraria, Research Funding. Kelly:Merck: Other: Scientific Steering Committee; Seagen: Other: Scientific Steering Committee.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal